Have any questions?
Get in touch-
Tel : +86 18355532477
-
Whatsapp : +8618355532477
-
Email : 247137166@qq.com
-
Skype : 247137166@qq.com
Have any questions?
Get in touchTel : +86 18355532477
Whatsapp : +8618355532477
Email : 247137166@qq.com
Skype : 247137166@qq.com
High-Entropy Alloys | laser cladding coating service
Apr 25 , 2021Definition:
High-Entropy Alloys (High-Entropy Alloys) referred to as HEAs, are composed of 5 or more main elements, and the atomic fraction of each main element accounts for 5%-35%. High-entropy alloys have many ideal properties. In the past concept, if more metal types are added to the alloy, the material will be embrittled, but high-entropy alloys are different from previous alloys, and there are many metals that do not embrittle.
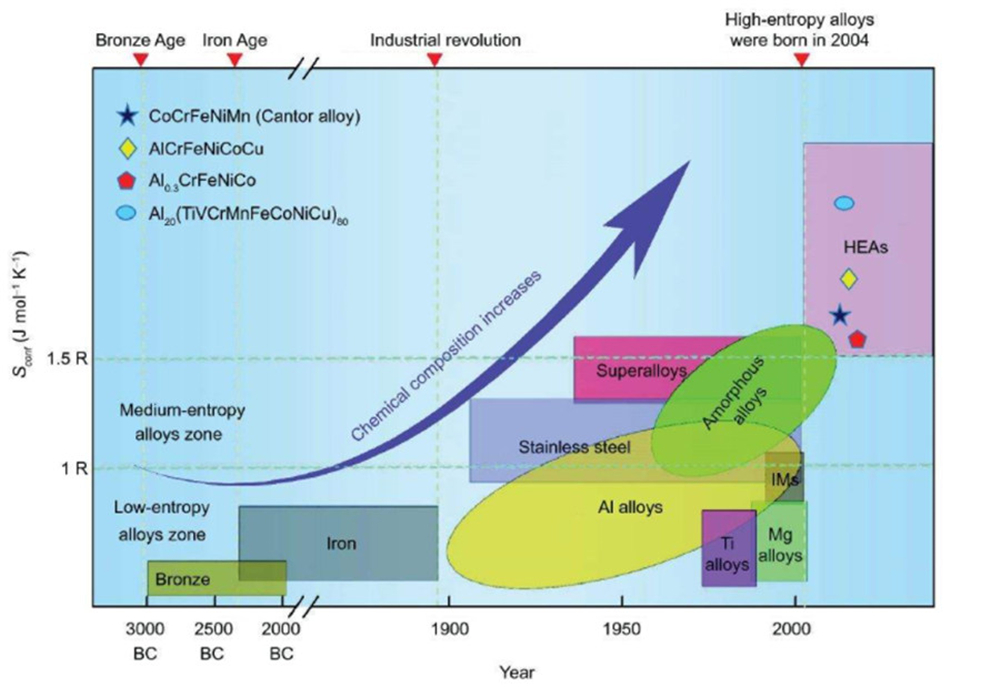
Entropy represents the degree of chaos in a system, the more chaotic the higher the entropy, the more orderly the lower the entropy. According to the second law of thermodynamics, all isolated systems will develop towards an increasing trend of entropy in nature.
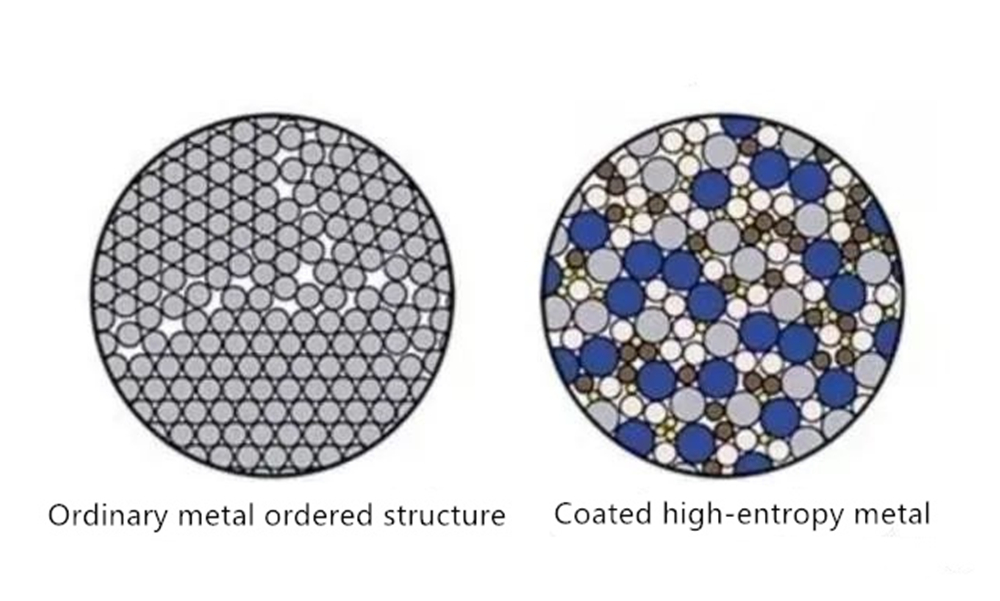
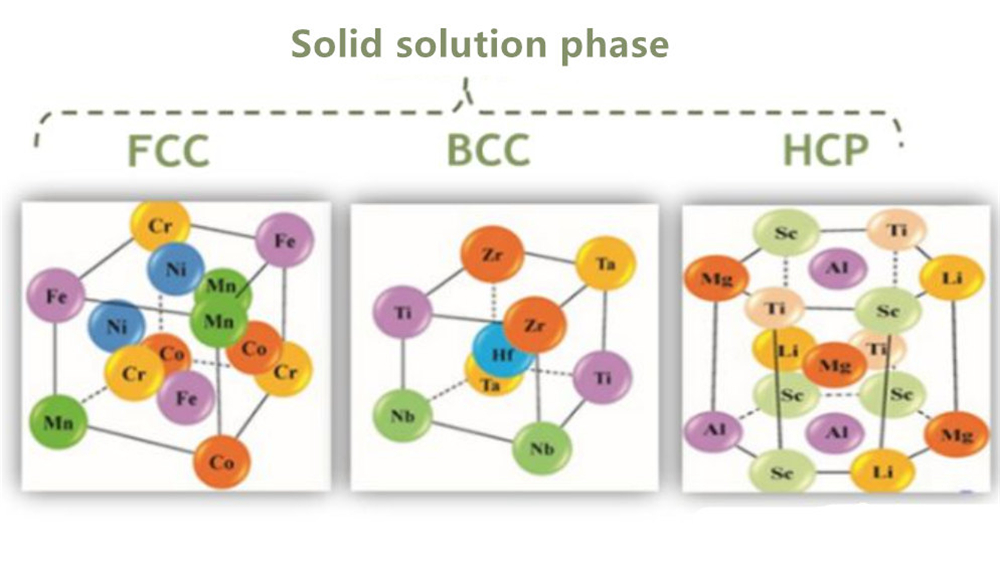
Although high-entropy alloys have many elements, they can often form a relatively simple phase structure after solidification. Randomly miscible solid solutions are typical structures of high-entropy alloys, including FCC, BCC, and HCP structures. Besides, the alloy will form part of the amorphous phase.
The core effects of high-entropy alloys:
High entropy effect
The high entropy effect is the iconic concept of HEAs. The ideal formation entropy and pure metal enthalpy (selected the enthalpy of formation of the IM compound) can be known that in a nearly equimolar alloy with 5 or more elements, it is more conducive to the formation of the SS phase rather than the IM compound. At this time, no special combination is considered, only the level of entropy and enthalpy is used to analyze the conventional SS phase and IM phase. The entropy value also only considers generating entropy. Although vibration, electrons, and magnetism also affect its entropy, the most important factor is still the structure of the alloy.
Lattice distortion
The severe lattice distortion is caused by the different atomic sizes in the high-entropy phase. The displacement of each lattice position depends on the atoms occupying that position and the type of atoms in the local environment. These distortions are much more serious than traditional alloys. The uncertainty of the position of these variable atoms leads to a higher enthalpy of formation of the alloy. Although physically, this can reduce the intensity of X-ray diffraction peaks, increase hardness, reduce electrical conductivity, and reduce the temperature dependence of the alloy. However, there is still a lack of systematic experiments to quantitatively describe the changes in these properties. For example, the shear modulus mismatch between the constituent atoms may also contribute to hardening; changes in local bonds may also change the electrical conductivity, thermal conductivity, and related electronic structure.
Slowly spread
In HEAs, diffusion is slow. This can be observed in the formation and microstructure of nanocrystalline and amorphous alloys.
"Cocktail" effect
The first "cocktail" effect was a phrase used by Professor S. Ranganathan. The original intention was "a pleasant, pleasant mixture". Later, it means a synergistic mixture, the final result is unpredictable and greater than the sum of the parts. This phrase describes three different alloy categories: bulk metallic glass, superelastic and superplastic metals, and HEAs. These alloys are multi-principal element alloys. The "cocktail" effect characterizes the structural and functional properties of the amorphous bulk metallic glass.
Unlike other "core effects", the "cocktail" effect is not a hypothesis and does not require proof. "Cocktail effect" means special material properties, usually due to unexpected synergy. Other materials can also be described in this way, including physical properties, such as close to zero coefficient of thermal expansion or catalytic response; functional properties, such as thermoelectric response or photoelectric conversion, ultra-high-strength, good fracture toughness; structural properties such as fatigue resistance or ductility. At this time, the properties of the material mainly depend on the material composition, microstructure, electronic structure, and other characteristics. The "cocktail" effect reveals the multi-element composition and special microstructure of MPEAs, which in turn produces unexpected non-linear results.
Applications:

Regardless of the type, the efficiency of the heat engine increases as the temperature rises. In power generation industries such as nuclear energy, coal-burning, and oil-fired, rising operating temperatures can reduce fuel consumption, pollution, and operating costs. In the jet engine industry, an increase in operating temperature can improve performance, such as heavier payload, greater speed, and greater range of combinations. At present, the development of the main components of the engine is still concentrated on Ni-based superalloy materials, but because its initial melting point is about 1300℃, the suitable temperature of nickel-based superalloy is only between 1160~1277℃. Therefore, the development of engine component materials with more excellent high-temperature performance becomes crucial. Tests show that the yield strength of these two refractory HEAs at 1600℃ exceeds 400MPa, which is much higher than the yield strength of Inconel 718 Ni-based superalloy at 1000℃ (less than 200MPa). The development of heat engines needs to further improve the high-temperature performance of engine component materials. Compared with Ni-based superalloys, HEAs have higher stability, lower cost and density, and positive lattice mismatch at high temperatures, which indicates that these alloys are likely to replace Ni due to their attractive high-temperature mechanical properties. Based superalloys are used as next-generation high-temperature materials.

The fracture of materials is often related to safety issues. Generally speaking, it can be divided into brittle and ductile fractures according to the failure strain. Brittle fracture has no signs of plastic deformation and usually occurs catastrophically. It is of great significance to develop new metal materials with excellent properties. It is reported that when the temperature drops from 298K to 77K, the fracture toughness of CrMnFeCoNi high-entropy alloy remains almost constant, while the fracture toughness of CrCoNi high-entropy alloy increases slightly. In these HEAs, there is no sharp ductile-brittle transition like many traditional alloys such as steel, amorphous alloys, magnesium alloys, porous metals, and nano-metals, which indicates that these alloys may be excellent candidates for applications under extremely cold conditions, such as, Used as materials for ship hulls, airplanes, and cryogenic storage tanks.
The WILA laser surface alloying method has prepared FeCoCrAlNi coating with good metallurgical bonding properties on 304 stainless steel. The test results show that the microhardness of FeCoCrAlNi coating is 3 times that of 304 stainless steel. In a 3.5% NaCl solution, its resistance Cavitation corrosion performance is about 7.6 times that of 304 stainless steel, and the current density is one order of magnitude lower than that of 304 stainless steel. Ye et al. used a laser surface alloying method to prepare a CrMnFeCoNi coating and conducted a potential dynamic polarization test in a 3.5% NaCl and 0.5mol/L H2SO4 solution. The results show that the corrosion resistance of the HEA coating is better than that of A36. For the steel matrix, the corrosion current is even lower than that of 304 stainless steel. As a newly developed multi-principal alloy, high-entropy alloy surpasses the design limit of traditional alloys based on a single majority of the main element and has the potential to improve corrosion resistance.
High-entropy alloys combine many excellent properties and can be applied in a wide range of industrial fields. High-entropy alloys have a strong ability to form amorphous, and some high-entropy alloys can form amorphous phases in the as-cast structure. To obtain an amorphous structure in traditional alloys, a great cooling rate is required to keep the structure with irregular distribution of liquid atoms to room temperature. The research of amorphous metals has only emerged in recent years. Because of the absence of dislocations in the structure, it has high strength, hardness, plasticity, toughness, corrosion resistance, and special magnetic properties, and is widely used. The preparation of amorphous high-entropy alloys will undoubtedly further expand the application fields of high-entropy alloys.
The development of high-entropy alloys:
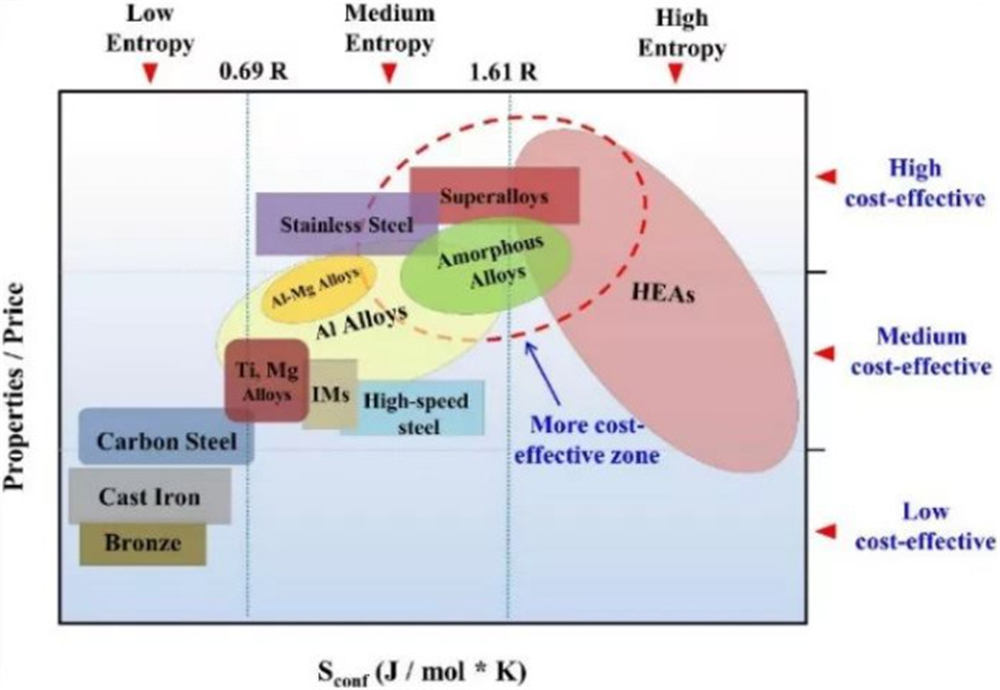
From traditional alloys to high-entropy alloys, the development of materials presents a trend of "entropy increase". However, the experimental results show that there is a nonlinear relationship between the mixing entropy and the properties of the material. In short, it is not that the higher the mixing entropy of the alloy material, the better the alloy performance; therefore, the blind pursuit of "high entropy" is not The performance of the material can be optimized infinitely. Besides, as the entropy of alloy materials increases, the number of alloy constituent elements also gradually increases. This means that the cost of the alloy will also increase accordingly. Therefore, blindly pursuing high mixing entropy will not improve the performance of the material, but will increase the cost of the alloy. According to the alloy "cost-effective" map obtained by statistics, it can be found that the most cost-effective area is not the high-entropy alloy area, but is located at the junction of the medium-entropy alloy and the high-entropy alloy, such as high-temperature alloys, amorphous alloys, stainless steel, and medium-entropy alloys. Etc. are more cost-effective. So this area will be a key area for future material development.
click here to leave a message